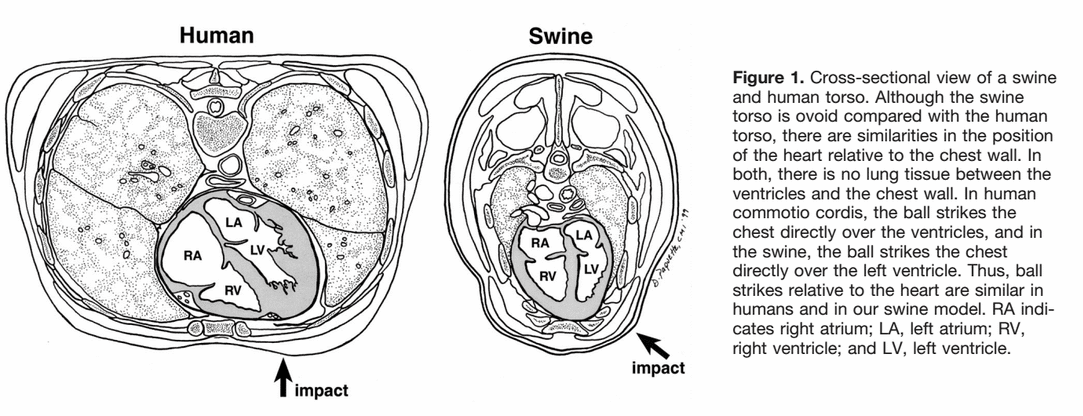
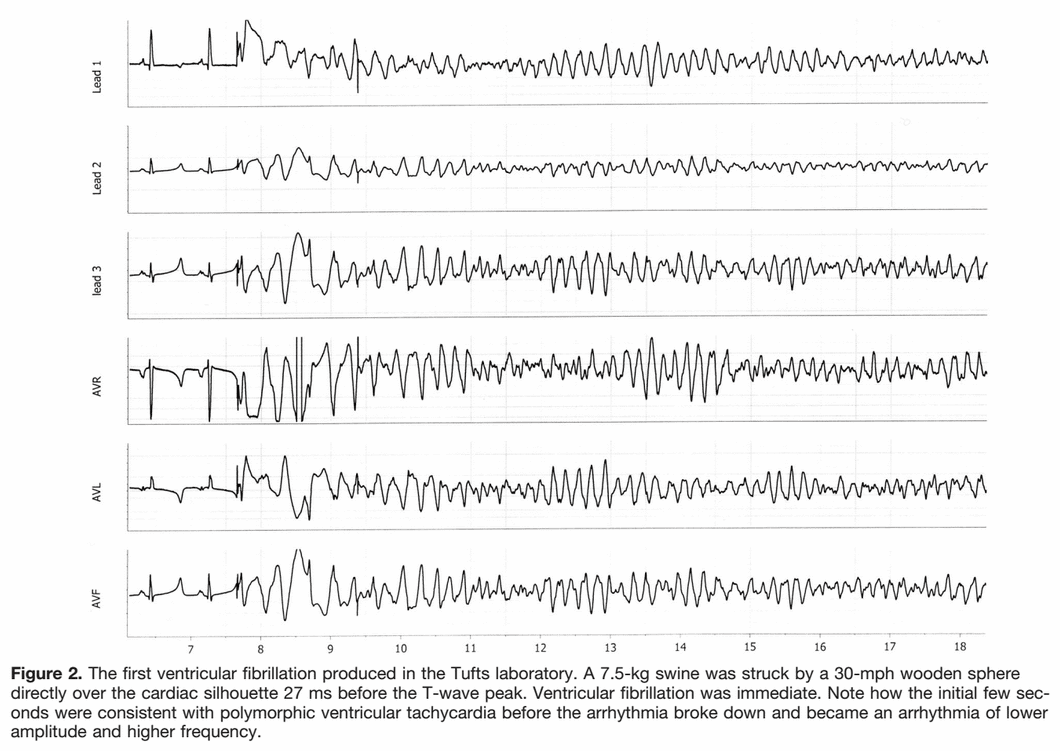
I have always been under the impression that commotio cordis was caused by an impact to the chest wall that was juuuust right in order to essentially "create" an R-on-T, resulting in vfib. For whatever reason the electrophysiology of the event was brought up in an AT class the other day, and the instructing GA didn't seem 100% on what he was saying about it, so I did some research (I don't remember what he said now). I'm reading that the impact occurs during the ~30milliseconds of the uphill of the T wave. This makes sense, as it would interrupt the repolarization of the ventricles, thus introducing vfib. But, what does this look like on an EKG?
I suppose my overall question is, could someone briefly explain the "why" behind commotio cordis? And if possible, describe what an EKG would show? (Yes, yes, I know we wouldn't see anything but a (hopefully) shockable rhythm on this kind of patient, but lets assume that patient happened to be wearing a Holter monitor or was hooked up to your LP when a baseball so perfectly entered through your back window).
Thanks guys.
I suppose my overall question is, could someone briefly explain the "why" behind commotio cordis? And if possible, describe what an EKG would show? (Yes, yes, I know we wouldn't see anything but a (hopefully) shockable rhythm on this kind of patient, but lets assume that patient happened to be wearing a Holter monitor or was hooked up to your LP when a baseball so perfectly entered through your back window).
Thanks guys.